
eLearning | Computer Based Training | Systems Simulation
In the world around us, a good understanding of what is happening at the atomic level forms the basis for truely understanding many important subjects such as electricity, magnetism and the properties of different engineering materials.
The CBT (Computer Based Training) package of interactive animations shown on this page can be used in the classroom to describe the structure of an atom, the different elements in the periodic table and explain the different types of chemical bonding (covalent bonds, ionic bonds and metallic bonds).
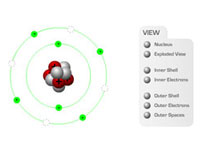
Using our CBT in the classroom, teachers or instructors can build up the structure of an atom, step-by-step, to describe the nucleus (protons and neutrons) and the electrons which occupy the inner and outer shells.
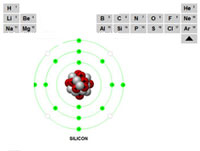
Using a section of the periodic table (showing the first 3 rows) teachers or instructors can select and describe each of the different elements.
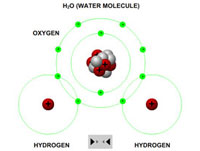
Teachers or instructors can clearly explain the process of covalent bonding using a variety of atoms (hydrogen, carbon and oxygen). These can be brought together to form a variety of different molecules and compounds (molecular hydrogen, molecular oxygen, water, methane and carbon dioxide).
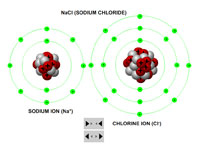
Using interactive models of sodium and chlorine atoms, teachers or instructors can demonstrate how an ionic bond is formed as the chlorine atom strips the sodium atom of its only valance electron, thereby turning the chlorine atom into negatively charged anion and the sodium atom into a positively charged cation.
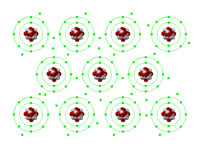
This interactive animation enables a clear explanation of how the electrons in metallic bonds become a "sea of electrons", shared between many metal atoms in the area, thereby giving metals their well-known properties such as malleability and conductivity.
If you wish to use any of our CBT within your training school, or require any bespoke scientific training material to be created, simply contact us!